Certificate


Certificate
Strengthened Business Based Competence

EN ISO 13485

Appointed as the star company of the area in 2011 (Ministry of Knowledge Economy)

Certificate of company laying research institute

Certificate of venture business

Goryeong factory received the certification of GMP for reagents, which are for the use of in-vitro diagnostic medical devices

Goryeong factory received the certification of GMP for in-vitro diagnostic devices
Acquired manufacture & sale system
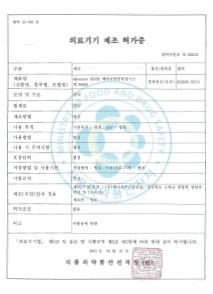
Licensed to manufacture items of medical device to KFDA (33 cases)
Possess technical excellence

Certified as a foreign-invested company of advanced technology Written decision (Ministry of Strategy and Finance) Appointed as a company possessing high-technology

Appointed as a company of advanced technology (Ministry of Knowledge Economy) Possess advanced detection technology
Bridgehead for the oversea markets

Certification of CE (BGMS)
-CareU™
-CareU™ SMART
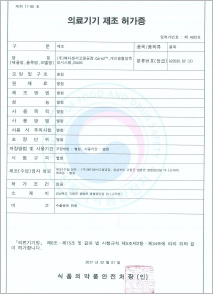
License for medical device manufacture (KFDA)
-CareU™
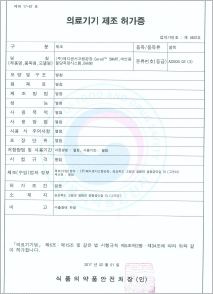
License for medical device manufacture (KFDA)
-CareU™ SMART
License for medical device manufacture (KFDA)
-CareU™ Test strip
License for medical device manufacture (KFDA)
-CareU™ SMART Test strip

certificate of CE (aQcare TRF)

certificate of CE (aQzen RDT-G (v1))

certificate of CE (aQzen RDT-G mini)

EN ISO 15197 : 2015
Other current status of certificate

